Medtronic receives FDA approval for renal denervation device
The company received approval for the hypertension treatment despite a mixed vote from an FDA advisory panel.

The company received approval for the hypertension treatment despite a mixed vote from an FDA advisory panel.
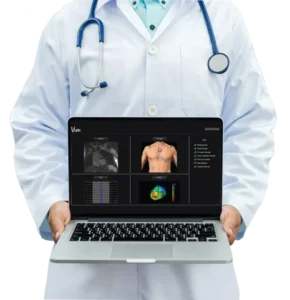
FORT MILL, SC / ACCESSWIRE / November 20, 2023 / Catheter Precision, Inc. (the “Company”) (NYSE American:VTAK), a pioneering medical technology firm headquartered in the United States, specializing in electrophysiology devices, has proudly disclosed the successful conclusion of the inaugural VIVO cases in the region of South-Eastern Europe. VIVO, the Company’s innovative 3D non-invasive imaging system, is used pre-procedurally to identify areas of interest for ventricular ablation and aid in pre-procedure planning to reduce procedure time and aid in procedural accuracy.
Medtronic (NYSE: MDT)+
said today that it’s received a CE mark for its PulseSelect pulsed field ablation system.