MedTech News
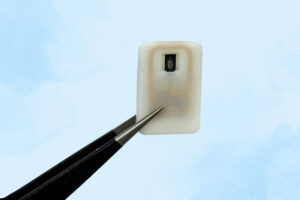
Implantable device could save diabetes patients from dangerously low blood sugar
The new implant carries a reservoir of glucagon that can be stored under the skin and deployed during an emergency — with no injections needed.
Boston Scientific wins expanded FDA nod for Farapulse PFA
Boston Scientific (NYSE: BSX)+
announced today that the FDA approved an expansion to the label of its Farapulse pulsed field ablation (PFA) system.

Intelligent wound dressing controls inflammation
Chronic wounds are a major medical challenge, burdening health care systems with billions of dollars in costs every year. Pioneer Fellow Börte Emiroglu is developing a new product: a selective, sponge-like hydrogel that reduces inflammatory signals and actively promotes healing.

Thermo Fisher’s NGS-Based Oncomine Dx Target Test Gains FDA Approval
FDA clears assay as companion diagnostic for ZEGFROVY™ and broad tumor profiling, advancing precision oncology diagnostics.

ReCerf Receives CE Mark, Expanding Access to Advanced Hip Resurfacing in Europe
Ceramic-based hip resurfacing implant gains European regulatory approval, offering patients a bone-preserving, metal-free alternative for hip joint restoration.

Camgenium’s medical device supports pregnant women at risk of pre-eclampsia
Camgenium, a leading medical device software company, has announced that its software product, ‘Reassure Pregnancy’, can improve outcomes for pregnant women at risk of pre-eclampsia through at-home monitoring.

Philips Secures FDA Clearance for SmartSpeed Precise, Advancing MRI Speed and Image Precision
Dual AI-Powered Imaging Software Set to Enhance Diagnostic Confidence and Workflow Efficiency in MRI
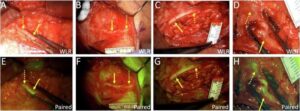
A new drug causes nerve tissue to emit light, enabling faster, safer surgery
When surgeons dissect tissue to remove a tumor or make a repair, they must work cautiously, relying on electrophysical monitors and their own anatomical knowledge to avoid cutting nerves, which could complicate the patient’s recovery.
