MedTech News

Simple blood test detects unique glycan linked to schizophrenia diagnosis
A group from Nagoya University in Japan has developed a simple, accurate, and sensitive method for measuring polysialic acid, a unique acidic glycan found in the brain. Polysialic acid fluctuates in the blood of patients with psychiatric disorders.

AI microscopy can improve parasite detection in health care
A new study from Karolinska Institutet shows that artificial intelligence (AI) combined with portable digital microscopy improves the detection of intestinal worm infections, so-called soil-transmitted helminth (STH) in resource-limited settings.
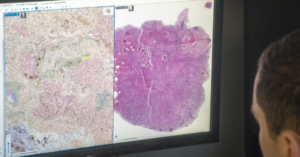
PathAI receives FDA clearance for AISight Dx platform
PathAI, a global leader in artificial intelligence (AI) and digital pathology solutions, has announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for AISight Dx—its digital pathology image management system—for use in primary diagnosis in clinical settings.

ReCerf Receives its CE Mark, Advancing Access to Ceramic Hip Resurfacing Across Europe
LEATHERHEAD, England, July 3, 2025 /PRNewswire/ — MatOrtho® is proud to announce that the ReCerf® Hip Resurfacing Arthroplasty (HRA) has received its CE mark, confirming compliance with European safety and performance standards. This important milestone expands access to hip resurfacing, enabling wider availability across the UK, Europe and internationally where CE marking supports market access.

ANA5 Funnel Catheter by ANACONDA Biomed Gets CE Mark, Unlocking New Era in Stroke Thrombectomy
In a recent conversation with MedTech Spectrum, Trent Reutiman, CEO of ANACONDA Biomed, shared insights into the company’s CE Mark approval for the ANA5 Funnel Catheter—a next-generation device designed to improve clot capture and procedural outcomes in acute ischemic stroke treatment.
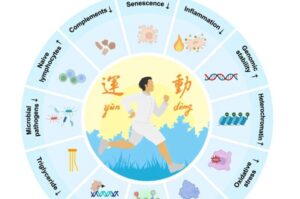
Mimicking the benefits of exercise with a single molecule
Capital Medical University, in collaboration with the Chinese Academy of Sciences, reports that betaine, a molecule produced in the kidney and enhanced through sustained exercise, operates as a potent inhibitor of inflammatory and aging-related pathways.
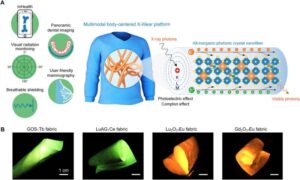
Wearable X-ray-detecting fabric offers a flexible alternative to current imaging tech
Since their discovery by Wilhelm Roentgen in 1895, X-rays have become a staple of modern medical care, from imaging teeth and broken bones to screening for the early signs of breast cancer.
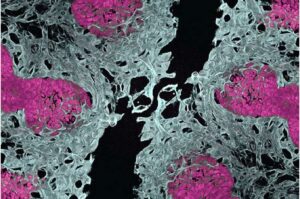
Scientists create first mini-lungs with built-in blood vessels, unlocking new insights for pulmonary vascular disease
UCLA researchers have successfully grown miniature lungs from stem cells—complete with their own functioning blood vessel networks.
