Imvaria and Darmiyan receive FDA authorizations for AI-enabled diagnostic tools
The de novo classifications cover software for detecting a lung condition and assessing dementia risk.

The de novo classifications cover software for detecting a lung condition and assessing dementia risk.

NeuroSigma today announced that the U.S. Food and Drug Administration (FDA) cleared use of NeuroSigma’s second-generation Monarch eTNS System for treating pediatric attention deficit hyperactivity disorder (ADHD)
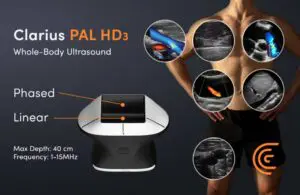
Clarius Mobile Health announced today that it received CE mark approval for its wireless handheld whole-body ultrasound scanner.

DermaSensor announced today that the FDA cleared its real-time, non-invasive, AI-powered skin cancer evaluation system.