
Masimo wins first FDA clearance for OTC medical fingertip pulse oximeter
The company’s OTC device, which costs nearly $300, will compete with consumer pulse oximeters that are widely available but lack FDA clearance.

The company’s OTC device, which costs nearly $300, will compete with consumer pulse oximeters that are widely available but lack FDA clearance.

The FDA created special controls that provide a path to market for other developers of over-the-counter devices.
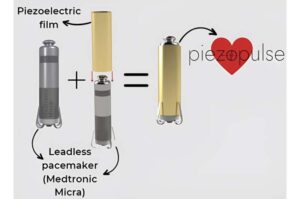
Heartbeats could one day help power something beyond hearts. A pacemaker is a device that helps regulate abnormal heart rates using electrical pulses. Leadless pacemakers, which are placed in the heart and don’t have wires like conventional transvenous pacemakers, are becoming more popular. However, their batteries only last five to 12 years, and it’s difficult to retrieve the device when the battery is drained.

SAN FRANCISCO, Feb. 14, 2024 (GLOBE NEWSWIRE) — ZKR Orthopedics, Inc., a clinical stage medical device company, announced that it has received Investigational Device Exemption (IDE) approval from the United States Food and Drug Administration (FDA) for a multicenter prospective clinical trial. The approved trial will evaluate the Company’s LIFT implant to treat later stage patellofemoral cartilage degeneration in adult patients.
Performing this test could help doctors prevent dysfunction that can occur when the right and left ventricles of the heart become imbalanced.