
Zimmer receives FDA clearance for robotic shoulder system
The clearance poises the orthopedics company to be first to market with robotic-assisted shoulder replacement surgery.

The clearance poises the orthopedics company to be first to market with robotic-assisted shoulder replacement surgery.

Allowance for U.S. Patent Application Serial Number 18/107,536 for Tenon’s Catamaran® SI Joint implant for stabilizing a dysfunctional sacroiliac (SI) joint.

For use in children weighing 10 kilograms or more with acute kidney injury (AKI) due to sepsis or a septic condition requiring kidney replacement therapy (KRT).
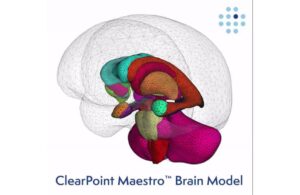
ClearPoint Neuro (Nasdaq:CLPT) announced that the FDA cleared its ClearPoint 2.2 software with integrated Maestro Brain Modeling.