
FDA clears new mammography system from Siemens Healthineers
Siemens Healthineers announced today that it received FDA 510(k) clearance for its MammoMat B.brilliant mammography platform.

Siemens Healthineers announced today that it received FDA 510(k) clearance for its MammoMat B.brilliant mammography platform.

New Indication for Treatment of Pulmonary Embolism Enhances Device Utility in Critical Medical Scenarios

This method of use patent award marks a major achievement for the Company and follows another recent patent covering our core technology.
The clearance adds to the list of devices the FDA has authorized this year with AI algorithms to detect health conditions.
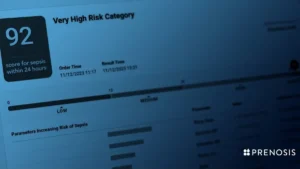
The CEO of Prenosis told MedTech Dive the company sees third-party validation as important, with the FDA having clarified that certain decision support tools should be regulated as medical devices.

The low-cost hardware outperforms state-of-the-art versions and could someday enable an affordable, in-home device for health monitoring.

TEG testing provides critical information that can help physicians improve hemostasis management for their patients