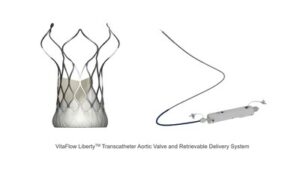
CardioFlow’s VitaFlow Liberty™ Granted EU CE-MDR Mark, Advancing Global Expansion Roadmap
SHANGHAI, June 17, 2024 /PRNewswire/ — MicroPort® CardioFlow Medtech Corporation (CardioFlow) (Stock Code: 02160.HK) recently announced that its self-developed second-generation transcatheter aortic valve implantation (TAVI) device, the VitaFlow LibertyTM Transcatheter Aortic Valve and Retrievable Delivery System (VitaFlow LibertyTM), has received EU CE-MDR certification. This certification highlights VitaFlow LibertyTM as a pioneering TAVI solution, that sets a new benchmark in transcatheter heart valve treatments.