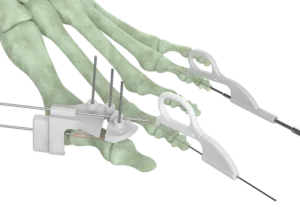
Forma Medical Granted FDA Clearance For Optimal Plating System Enabling a Multi-Faceted Growth Strategy for MIS Forefoot Portfolio
CAMP HILL, Pa.–(BUSINESS WIRE)–Forma Medical Inc., a leading innovator in medical device technologies, is thrilled to announce the FDA 510(k) clearance for its groundbreaking OptimalMTP® Plating System. This regulatory milestone marks a significant leap forward in MIS (Minimally Invasive Surgery), ushering in a new era of precision and efficiency dedicated to foot and ankle surgery, one of the fastest growing segments in the medical device space.