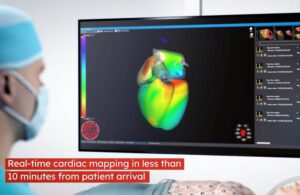
Corify wins CE mark for cardiac mapping tech
Corify Care announced today that it received CE mark approval under the new medical device regulation (MDR) for its Acorys cardiac mapping system.

Corify Care announced today that it received CE mark approval under the new medical device regulation (MDR) for its Acorys cardiac mapping system.
CorVascular announced today that the FDA granted clearance for its VasoGuard V-Series portfolio of devices.

EYSINS, Switzerland, July 29, 2024 /PRNewswire/ — AliveDx (“Company”) is proud to announce it has received IVDR CE mark for its groundbreaking microarray immunoassay, designed for the detection of a specific IgE directed to a protein allergen in human serum. This is the Company’s first product for the evaluation of allergies, enhancing its portfolio of products beyond autoimmunity.