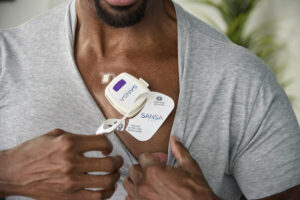
U.S. Food and Drug Administration Gives Huxley Medical 510(k) Clearance for SANSA Home Sleep Apnea Test
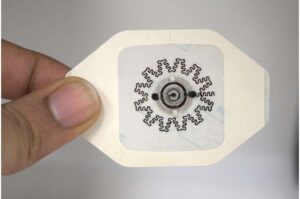
ATLANTA, Aug. 7, 2024 /PRNewswire/ — Huxley Medical, a developer of technologies that streamline cardiopulmonary care, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its groundbreaking, chest-worn, sleep apnea diagnostic patch, SANSA.