
CereVasc wins FDA breakthrough nod for eShunt system
CereVasc announced today that it received FDA breakthrough device designation for its eShunt system for treating normal pressure hydrocephalus (NPH).

CereVasc announced today that it received FDA breakthrough device designation for its eShunt system for treating normal pressure hydrocephalus (NPH).

Neuros Medical announced today that it received FDA approval for its Altius direct electrical nerve stimulation system.
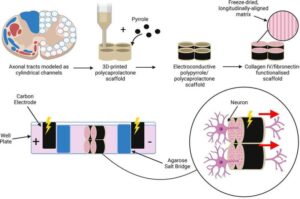
A research team at RCSI University of Medicine and Health Sciences has developed a new implant that conveys electrical signals and may have the potential to encourage nerve cell (neuron) repair after spinal cord injury.

INDIANAPOLIS, Aug. 28, 2024 /PRNewswire/ — Prevounce Health, a leading provider of remote care management software, devices, and services, announces the launch of its first blood oxygen device for remote patient monitoring (RPM): Pylo OX1-LTE.

The new ACURATE Prime valve system is indicated to restore function and normal blood flow through a narrowed aortic valve in low, intermediate and high-risk patients with severe aortic stenosis.