
Abbott launches Lingo over-the-counter CGM biosensor in the U.S.
Abbott (NYSE: ABT)+
today announced a major product launch, broadening the population that can utilize its continuous glucose monitoring (CGM) technology.

Abbott (NYSE: ABT)+
today announced a major product launch, broadening the population that can utilize its continuous glucose monitoring (CGM) technology.

CENTER VALLEY, Pa., Sept. 5, 2024 /PRNewswire/ — Odin Medical Ltd., an Olympus Corporation company, has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the first cloud-based Artificial Intelligence (AI) technology designed to assist gastroenterologists in detecting suspected colorectal polyps during colonoscopy procedures, the CADDIE™ computer-aided detection (CADe) device.
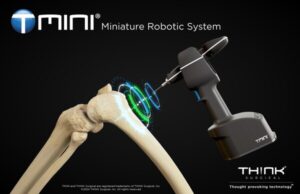
FREMONT, Calif., Sept. 5, 2024 /PRNewswire/ — THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use with the Persona® The Personalized Knee System® from Zimmer Biomet.