FDA clears Femasys’ FemChec diagnostic device
Femasys today announced it received FDA 510(k) clearance for its FemChec contrast-generating device that checks fallopian tubal status.

Femasys today announced it received FDA 510(k) clearance for its FemChec contrast-generating device that checks fallopian tubal status.
DeepWell Digital Therapeutics (DTx) has won FDA 510(k) clearance of technology to be used in immersive media like video games for stress reduction and as an adjunctive treatment for high blood pressure.
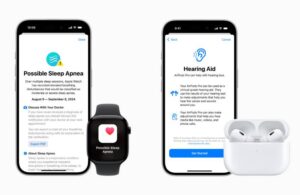
Apple today unveiled new sleep and hearing health features for its Apple Watch and AirPods Pro 2, expanding its health metrics offerings.

MGI Tech Co., Ltd. (“MGI”), a company committed to building core tools and technologies that drive innovation in life science, today announced the global rights to commercialize and distribute the new sequencing products CycloneSEQ-WT02* and CycloneSEQ-WY01*