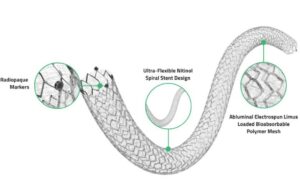
Medinol has first human implant of drug-eluting peripheral stent in Australia
Medinol today announced the successful first-in-human implantation of its ChampioNIR drug-eluting peripheral stent.

Medinol today announced the successful first-in-human implantation of its ChampioNIR drug-eluting peripheral stent.
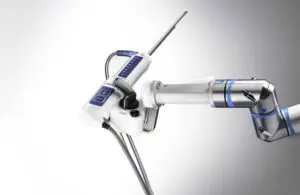
Procept BioRobotics (Nasdaq:PRCT) announced today that the FDA approved an investigational device exemption (IDE) trial for its Aquablation therapy.

CHICAGO, Oct. 3, 2024 /PRNewswire/ — Amphix Bio, a company developing a new class of regenerative medicine therapies, announced today it has received a Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for a drug-device combination product for bone regeneration. The designation covers the use of the therapeutic device to treat degenerative disc disease with transforaminal lumbar interbody fusion (TLIF) procedures.