Corin wins FDA clearance for new femoral stem
Corin announced that it received FDA 510(k) clearance for its Icona femoral stem implant for total hip arthroplasty (THA).
Corin announced that it received FDA 510(k) clearance for its Icona femoral stem implant for total hip arthroplasty (THA).
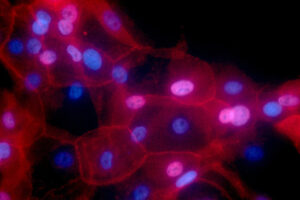
The drug-device combination developed by MIT spinout Lumicell is poised to reduce repeat surgeries and ensure more complete tumor removal.
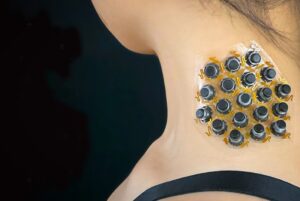
Device delivers various sensations, including vibrations, pressure and twisting

BOSTON, Nov. 6, 2024 /PRNewswire/ — Zeta Surgical announced today that its Zeta Navigation System has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use with expanded instruments and enhanced hospital connectivity.