Johnson & Johnson wins FDA IDE for Ottava surgical robot
Johnson & Johnson MedTech announced today that the FDA granted its Ottava surgical robot investigational device exemption (IDE).

Johnson & Johnson MedTech announced today that the FDA granted its Ottava surgical robot investigational device exemption (IDE).

Nevro (NYSE:NVRO) announced today that it received CE mark in Europe for its HFX iQ spinal cord stimulation (SCS) system.
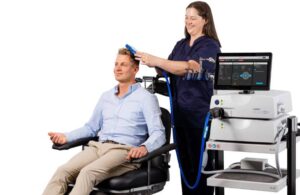
Magstim announced today that the FDA cleared its next-generation Horizon Inspire system for delivering transcranial magnetic stimulation (TMS).
iCad (Nasdaq:ICAD) announced today that the FDA cleared its ProFound Detection version 4.0 for digital breast tomosynthesis (DBT).
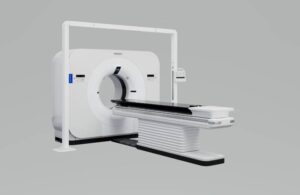
Philips (NYSE:PHG) announced today that the FDA granted 510(k) clearance for its new detector-based spectral computed tomography (CT) radiotherapy solution.

Fibrin sheath formation is a challenge for physicians using CVCs, as it blocks blood flow and makes CVCs less effective; the new AstutePlus prevents fibrin sheath formation.

MedTech startup Accunea has developed RenoSure, a new device that has been designed to ‘transform’ kidney care according to the company, and increase the number of organs available for transplantation.

MediKraft SealBase is a surface-treated medical paper that the company says is designed to meet the growing needs of the medical packaging industry.