
Dean Kamen, insulin pump pioneer, wants to shake up diabetes tech
Kamen and Sequel Medtech CEO Alan Lotvin say they are offering greater precision in insulin delivery with new pump technology in the Twiist device.

Kamen and Sequel Medtech CEO Alan Lotvin say they are offering greater precision in insulin delivery with new pump technology in the Twiist device.
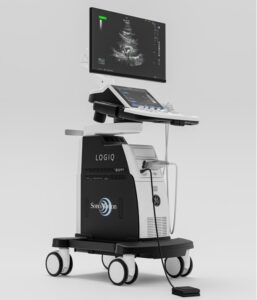
SAN MATEO, Calif., Nov. 13, 2024 /PRNewswire/ — SonoMotion, a medical device company developing non-invasive solutions for kidney stones, announced today that the FDA has granted de novo clearance for the Company’s Stone Clear™ device for the anesthesia-free treatment of post-lithotripsy kidney stone fragments.
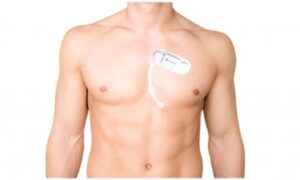
LAUSANNE, Switzerland, Nov. 13, 2024 /PRNewswire/ — SmartCardia has received FDA clearance for Mobile Outpatient Cardiac Telemetry (OCT/MCT) for its 7-lead live ECG monitoring patch and cloud platform. SmartCardia’s 7L patch is easy-to-wear, cable-free, waterproof and can be used for continuous monitoring for up to 14 days