
FDA clears Nonin’s Tru02 over-the-counter fingertip pulse oximeter
Nonin Medical has won FDA 510(k) clearance for its first over-the-counter fingertip pulse oximeter, the TruO2 OTC.

Nonin Medical has won FDA 510(k) clearance for its first over-the-counter fingertip pulse oximeter, the TruO2 OTC.

IRVINE, Calif., Dec. 2, 2024 /PRNewswire/ — Mentor Worldwide LLC, the number one global brand in breast aesthetics, and part of Johnson & Johnson MedTech, today announced the U.S. Food and Drug Administration (FDA) approved MENTOR™ MemoryGel™ Enhance Breast Implants for primary and revision reconstruction breast surgery in post-mastectomy women.
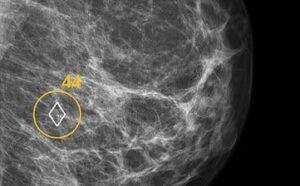
CHICAGO, Dec. 2, 2024 /PRNewswire/ — ScreenPoint Medical is showcasing a new FDA clearance for innovative new capabilities of its industry leading Breast AI Transpara here at the 110th Annual Radiological Society of North America (RSNA) meeting, December 1-4, 2024 (South Hall #5316)
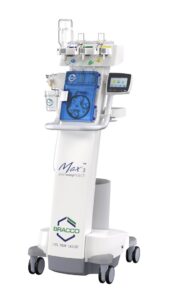
PRINCETON, N.J., Dec. 2, 2024 /PRNewswire/ — Bracco Diagnostics Inc., the U.S. subsidiary of Bracco Imaging S.p.A., a leading global company in the diagnostic imaging business, along with ulrich GmbH & Co. KG, a renowned German medical device manufacturer specializing in contrast media injectors and spinal implants, announced today the clearance of the Bracco branded Max 3™, a Rapid Exchange and Syringeless Injector for use in magnetic resonance imaging (MRI) procedures.

PLEASANTON, Calif., Dec. 2, 2024 /PRNewswire/ — Movano Health (Nasdaq: MOVE), a pioneer in health technology, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the pulse oximeter in its EvieMED Ring.