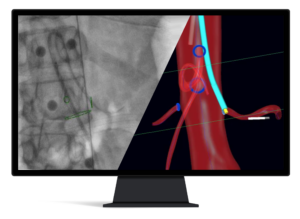
Centerline Biomedical receives FDA 510(k) clearance for new IOPS® Guidewire Handle
CLEVELAND, Dec. 4, 2024 /PRNewswire/ — Centerline Biomedical, Inc. (“Centerline”), an innovative leader in cardiovascular navigation and visualization systems, announced that the company has received US Food and Drug Administration (FDA) 510(k) clearance for its new IOPS Guidewire Handle