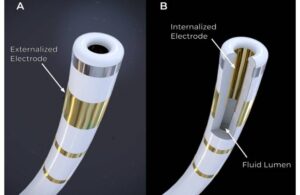
FDA grants breakthrough nod to pulsed field ablation tech from Farapulse founder’s Field Medical
Field Medical announced today that it received FDA breakthrough device designation for its FieldForce pulsed field ablation (PFA) system.

Field Medical announced today that it received FDA breakthrough device designation for its FieldForce pulsed field ablation (PFA) system.

A new medical innovation, developed in the UK by Europlaz, has the potential to help improve neonatal care and save the lives of more babies born prematurely or in distress according to the company.
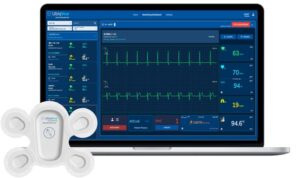
MILPITAS, Calif., Dec. 5, 2024 /PRNewswire/ — LifeSignals, Inc. today announced that the UbiqVue 2A Multiparameter System has received EU MDR Certification, marking another significant milestone in deploying continuous wireless patient monitoring for population health management following FDA 510(k) Clearance last month.
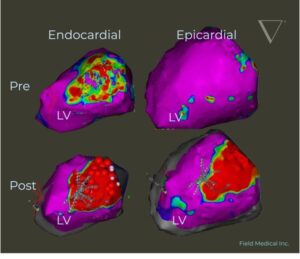
First and only contact force pulsed field ablation (PFA) system engineered to revolutionize care for the hundreds of thousands at risk of death from ventricular tachycardia (VT).

Implantable device works like a tree branch to grab and fling proteins