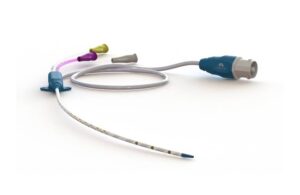
Lungpacer Medical wins FDA premarket approval for AeroPace neurostim system
Lungpacer Medical announced that it received FDA premarket approval for its flagship AeroPace neurostimulation system.

Lungpacer Medical announced that it received FDA premarket approval for its flagship AeroPace neurostimulation system.
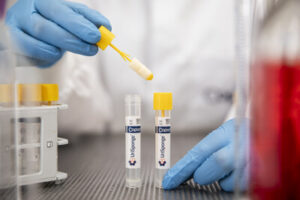
The solution offers clinical laboratories and healthcare providers a user-friendly, reliable, and cost-effective way to improve urine specimen collection, preservation, and transport.
LINKÖPING, Sweden, Dec. 6, 2024 /PRNewswire/ — SyntheticMR, leader in quantitative imaging software, is pleased to announce that its SyMRI 15 solution has obtained 510(k) clearance from the U.S. Food and Drug Administration (FDA) on diagnostic image replacement of conventional images.