
MannKind reports positive data for inhaled insulin for children
MannKind today announced six-month results from its Phase 3 INHALE-1 study of Afrezza insulin inhalation powder in children.

MannKind today announced six-month results from its Phase 3 INHALE-1 study of Afrezza insulin inhalation powder in children.

DÜSSELDORF, Germany, Dec. 16, 2024 /PRNewswire/ — Gerresheimer, an innovative system and solution provider and a global partner for the pharma, biotech and cosmetics industries, announces that the US Food and Drug Administration (FDA) granted SQ Innovation Tentative Approval for Lasix ONYU for the home treatment of fluid overload in congestive heart failure.

The Osseofit devices are intended to match patients’ shoulder bone anatomy and preserve healthy bone in total shoulder replacement procedures.

HeartBeam (Nasdaq:BEAT) announced today that it received FDA 510(k) clearance for its comprehensive arrhythmia assessment system.

Withings Health Solutions announced today that it received FDA clearance for BPM Pro 2, a first-of-its-kind cellular blood pressure monitor.
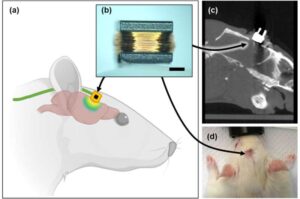
A research team led by Prof. Kim So-hee from the Department of Robotics and Mechanical Electronics, DGIST, has developed a technology that enables precise brain stimulation using a coil small enough to be implanted in the body. It is expected to be utilized as an electronic medicine for brain neurological disorders that require long-term treatment due to its ability to significantly improve safety and effectiveness with fewer side effects compared to existing technologies.
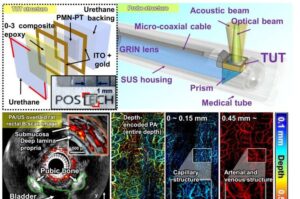
A research team has developed the world’s first high-performance photoacoustic endoscopy based on a transparent ultrasonic transducer. Their findings were recently published in the journal Science Advances.
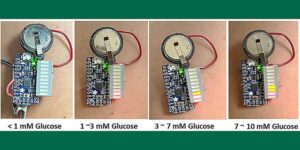
Millions of people with diabetes track their glucose levels daily using finger-stick devices that draw and analyze their blood. But what if they could monitor it with just a sweat sensor?
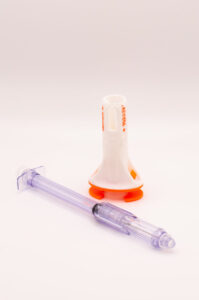
VANCOUVER, BC, Dec. 16, 2024 /PRNewswire/ — Zephyrus Innovations (Zephyrus), a privately-owned medical device company designing and manufacturing safety syringes and Closed System Transfer Devices (CSTDs), announced its latest product, VaporShield, the world’s first injectable closed system transfer device (CSTD), at the Partnership Opportunities in Drug Delivery (PODD) conference in Boston, MA last month.

ALBUQUERQUE, N.M., Dec. 16, 2024 /PRNewswire/ — Gastro Concepts, a team committed to advancing safety and efficiency in gastroenterology, today announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its groundbreaking Air Assist™ device.