
FDA clears Nalu peripheral nerve stim tech for whole-body MRI
Nalu Medical announced today that the FDA cleared expanded labeling of its peripheral nerve system to include whole-body MRI use.

Nalu Medical announced today that the FDA cleared expanded labeling of its peripheral nerve system to include whole-body MRI use.

President Donald Trump has returned to office and swiftly enacted a series of executive orders that could impact the medtech industry.

LONDON and MUNICH and NEW DELHI, Jan. 21, 2025 /PRNewswire/ — Datar Cancer Genetics (DCG) today announced the launch of Target-MRD, an advanced molecular residual disease (MRD) monitoring blood test for solid organ cancers. Target-MRD is a blood test based on tumor-agnostic next-generation sequencing (NGS) and customized, tumor-informed droplet digital PCR (dd-PCR) assay.

SUNNYVALE, Calif., Jan. 21, 2025 /PRNewswire/ — Inflammatix, a pioneering host response diagnostics company, today announced that the U.S. Food and Drug Administration (FDA) has granted marketing authorization for the TriVerity™ Test System (TriVerity), a first-in-class molecular test for patients with suspected acute infection or sepsis. Using precise measurements of a patient’s immune response, TriVerity combines highly accurate bacterial-viral infection scoring with an all-cause illness severity risk evaluation, giving clinicians a rapid and holistic snapshot of a patient’s status.

HOUSTON, Jan. 21, 2025 /PRNewswire/ — IntelliGenome, an innovative molecular diagnostic solutions provider, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to its CRISPR-Tuberculosis (TB) Blood Test. This test is the first qualitative real-time polymerase chain reaction (PCR) assay to combine CRISPR technology, designed to detect Mycobacterium tuberculosis (Mtb) cell-free DNA in human serum and EDTA plasma.
PALO ALTO, Calif., Jan. 21, 2025 /PRNewswire/ — Glooko, Inc., a global integrated digital health company connecting patients, providers, biopharma, and medical device partners, today announced that its Glooko XT solution has been approved for reimbursement by the Haute Autorité de Santé (HAS) and the Commission Nationale d’Évaluation des Dispositifs Médicaux et des Technologies de Santé (CNEDiMTS) in France.

SARATOGA, Calif., Jan. 21, 2025 /PRNewswire/ — CapsoVision, Inc., a leader in innovative endoscopic capsule technology, today announced that its award-winning product, the CapsoCam Plus® capsule endoscopy system, has received U.S. Food & Drug Administration (FDA) clearance for remote ingestion.
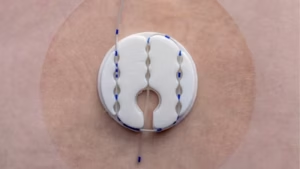
BETHLEHEM, Pa., Jan. 21, 2025 /PRNewswire/ — B. Braun Medical Inc. (B. Braun), a leader in smart infusion therapy and pain management, announced today the launch of its new Clik-FIX® Epidural/Peripheral Nerve Block (PNB) Catheter Securement Device, the latest addition to the Clik-FIX Family of Catheter Securement Devices.

A research team led by Dr. Youngdo Jeong of the Center for Advanced Biomolecular Recognition at the Korea Institute of Science and Technology (KIST), in collaboration with Professor Seok-Ho Kang’s team from the Department of Urology, Korea University College of Medicine, has developed a urine-based diagnostic kit for bladder cancer that can be conveniently used at home