Neuvotion Receives FDA Clearance for NeuStim™ Providing Non-Invasive, High-Resolution Stimulation for the Hand After Stroke or Spinal Cord Injury
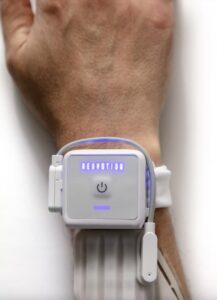
STAMFORD, Conn., Feb. 11, 2025 /PRNewswire/ — Neuvotion, Inc. is an early-stage medical device company developing AI-driven neuromodulation technologies and products for use in the neurorehabilitation, brain-computer interface (BCI), and physical therapy markets. Neuvotion has received FDA 510(k) clearance for their first product, NeuStim™, a non-invasive, surgery-free wearable that electrically stimulates muscles dynamically and with high-precision.