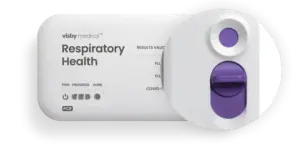
Visby Medical™ Receives FDA Clearance and CLIA Waiver for Point-of-Care Respiratory Health Test
SAN JOSE, Calif., Feb. 26, 2025 /PRNewswire/ — Visby Medical™ announced today that it has received 510(k) clearance and was granted a CLIA waiver from the U.S. Food and Drug Administration (FDA) for its point-of-care respiratory health test. The Visby Medical Respiratory Health Test is a rapid polymerase chain reaction (PCR) test that detects and differentiates between upper respiratory infections caused by influenza (Flu) A & B, and SARS-CoV-2 (COVID-19). This multiplexed molecular device is the first handheld test to receive this designation after being granted Emergency Use Authorization (EUA) in December 2022.