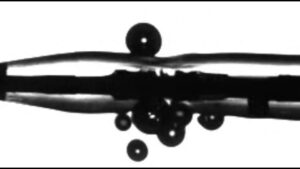
FDA clears Boston Scientific Bolt IVL system
Boston Scientific (NYSE: BSX) has received FDA clearance for its Bolt intravascular lithotripsy (IVL) system, according to the FDA’s 510(k) database.

Boston Scientific (NYSE: BSX) has received FDA clearance for its Bolt intravascular lithotripsy (IVL) system, according to the FDA’s 510(k) database.
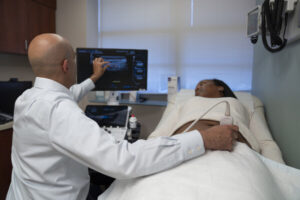
WASHINGTON, March 31, 2025 /PRNewswire/ — MedStar Georgetown University Hospital is the first hospital in the Washington, D.C. region to offer bedside intestinal ultrasound (IUS) to monitor inflammation in IBD patients. To assess chronic inflammatory diseases of the intestines, like Crohn’s disease and ulcerative colitis, patients are traditionally monitored with blood tests, stool studies, MRI or CT scans, and colonoscopies. Those kinds of tests can require bowel preparation, sedation, anesthesia, and radiation.
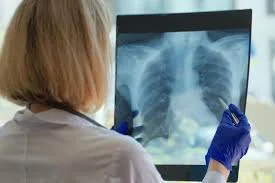
PARIS, March 31, 2025 /PRNewswire/ — AZmed, one of the leading AI companies in medical imaging, today announced that it has received two new U.S. Food and Drug Administration (FDA) clearances for its AI tool AZchest. The clearances include applications intended to assist radiologists in the interpretation and detection of chest X-rays for lung nodules and triage capabilities for pneumothorax and pleural effusion.