
Abbott launches next-gen delivery system for Proclaim DRG neurostim
Abbott (NYSE: ABT) announced today that it launched a next-generation delivery system for its neuromodulation business.

Abbott (NYSE: ABT) announced today that it launched a next-generation delivery system for its neuromodulation business.
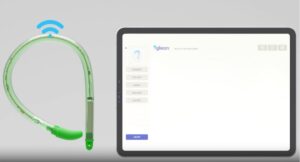
Bright Uro announced today that it received FDA 510(k) clearance for its Glean urodynamic analyzer system.

FDA Chief Medical Officer Hilary Marston said she’s no longer with the agency as the Trump Administration started layoffs in the U.S. Department of Health and Human Services (HHS).

Tiny device can be inserted with a syringe, then dissolves after it’s no longer needed
A team of Northwestern scientists spanning disciplines have developed new technology that could lead to the creation of a rapid point-of-care test for HIV infection competitive with traditional lab-based HIV testing in a fraction of the time and without the need for a stressful wait while results are processed or confirmed in a clinical laboratory.
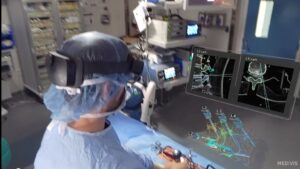
NEW YORK, April 2, 2025 /PRNewswire/ — Medivis Inc., a pioneer in surgical intelligence, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its groundbreaking Spine Navigation platform. This milestone marks a significant advancement in surgical technology, utilizing AR and AI to empower surgeons with holographic navigation across open and minimally invasive spine procedures. Alongside the FDA clearance, Medivis is announcing the commercial launch of Spine Navigation, making it available to hospitals and ambulatory surgical centers nationwide.