The Gut Microbiome Could Help Our Bodies Fight Cancer
The body’s home-grown microbiota and bile acids could help boost the immune system to suppress tumor growth.

The body’s home-grown microbiota and bile acids could help boost the immune system to suppress tumor growth.

Researchers from Children’s Hospital of Philadelphia (CHOP) and the Perelman School of Medicine at the University of Pennsylvania (Penn Medicine) have successfully employed an algorithm to identify potential mutations which increase disease risk in the noncoding regions of our DNA, which make up the vast majority of the human genome.
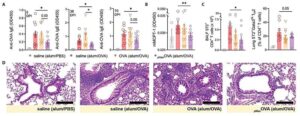
For millions of people around the world, asthma is more than just a breathing problem—it is a chronic and often debilitating condition caused by the immune system’s exaggerated response to harmless airborne particles.
High-intensity electrical pulses have been medically used to destroy tumors while sparing healthy tissue. But lower-intensity pulses may have a different effect—they reshape the battlefield, making tumors more vulnerable to the body’s own defenses.

New implants aim to enhance outcomes in foot and ankle surgeries through advanced material technology.
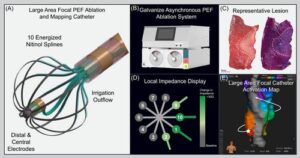
CardioFocus announced the completion of successful clinical cases with its QuickShot Nav large-area focal pulsed field ablation (PFA) catheter.

Precision Neuroscience announced today that it received FDA 510(k) clearance for its Layer 7 cortical interface for BCI technology
Adagio Medical (Nasdaq:ADGM) announced today that it received FDA breakthrough device designation for its vCLAS cryoablation system

SAN FRANCISCO, April 17, 2025 /PRNewswire/ — Solo Pace Incorporated, an emerging medical technology company, announced both FDA Clearance and First-In-Human use of their SoloPace Control System for temporary pacing in Transcatheter Aortic Valve Replacement (TAVR) procedures. With standardized workflows, the Control System is engineered to improve TAVR temporary pacing efficiency and reduce patient risks. Initial cases were completed this month at Scripps Clinic by Chief of Cardiology, Paul Teirstein, MD and Curtiss Stinis, MD.

Harvard’s Wyss Institute — a top medtech research institute in the U.S. — has received Stop Work Orders on three government contracts.