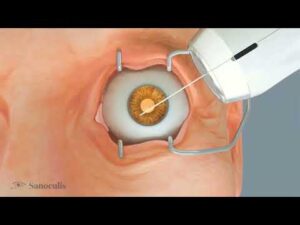
Sanoculis Receives CE Mark for MINT® Product for the Treatment of Glaucoma
TEL AVIV, Israel, April 17, 2025 /PRNewswire/ — Sanoculis Ltd., a company specializing in ophthalmic medical device technologies, today announced that it has received CE Mark approval under the Medical Device Regulation (MDR) in the European Union (EU) for its MINT® (Minimally Invasive Nasal Trabeculostomy) product—an innovative, stent-free technology platform for the treatment of adult patients undergoing glaucoma angle surgery.
