
IdentifySensors Advances AI-Powered Diagnostic Tech with FDA Submission
AI-driven system targets real-time, lab-quality disease detection at the point of care

AI-driven system targets real-time, lab-quality disease detection at the point of care
Medtronic (NYSE: MDT)+
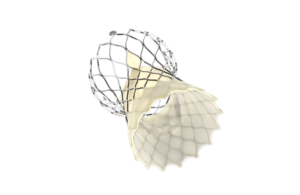
this week announced it received CE Mark approval for an expanded indication of its Evolut Pro+ and Evolut FX TAVI systems, allowing the devices to be used in “redo TAVI” procedures for patients with failing transcatheter aortic valves, regardless of the original manufacturer.

Natus Medical yesterday announced that it launched BrainWatch, a point-of-care EEG solution driven by AI.

Johnson & Johnson MedTech (NYSE: JNJ)+
announced today that it launched the SoundStar Crystal ultrasound catheter.
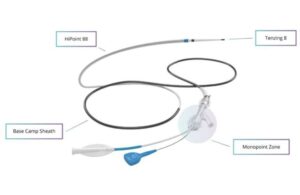
Route 92 Medical announced today that it received FDA 510(k) clearance for its HiPoint reperfusion system.