Johnson & Johnson MedTech launches newest Ethicon surgical stapler
Johnson & Johnson MedTech (NYSE: JNJ)+
today announced the U.S. launch of its Ethicon 4000 surgical stapler.
Johnson & Johnson MedTech (NYSE: JNJ)+
today announced the U.S. launch of its Ethicon 4000 surgical stapler.

Smith+Nephew (NYSE: SNN)+
this week announced it launched a new medial stabilized insert for its Legion Total Knee System (TKS).
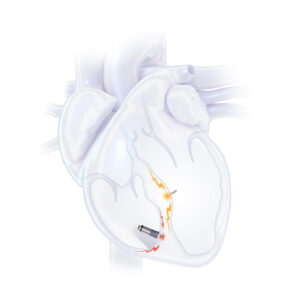
SUNNYVALE, Calif., June 10, 2025 /PRNewswire/ — In a major milestone for heart failure treatment, the first commercial patients in the U.S. have been successfully implanted with the FDA-approved WiSE® System—marking the beginning of a new chapter in leadless left ventricular endocardial pacing (LVEP) for the treatment of heart failure.
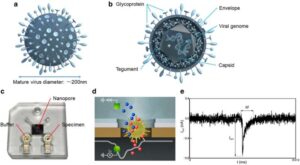
In a study published in PNAS Nexus, Noriyasu Hashida and colleagues designed a test that confirms the presence of live virus by pushing particles through a nanopore, one at a time, and measuring their electrical conductivity, which varies with size and surface charge as well as the unique molecular structure of the virus.
New digital twin technology that allows a University of Virginia-developed artificial pancreas system to adapt to users’ changing needs—and lets users adjust the settings—has been shown to improve type 1 diabetes control, according to a study published in npj Digital Medicine.
Breakthrough Non-Invasive Therapy Installed with Support from Li Ka Shing Foundation to Advance Research and Patient Care

A team of scientists and biomedical engineers developing pioneering technology designed to treat threatened miscarriage has secured 1 million GBP in Invention for Innovation (i4i) funding from the National Institute for Health and Care Research (NIHR) for its first clinical trial.

Mediplus has announced the release of new sizes of the POPY (Pelvic Organ Prolapse pessary) product range. The company is introducing five additional sizes to complement its existing products, offering a more precise fit and enhanced treatment options for women suffering from pelvic organ prolapse.
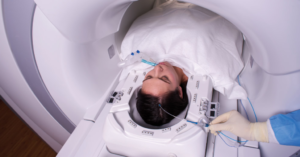
Medtronic has announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Visualase V2 MRI-Guided Laser Ablation System. This milestone brings important capital system enhancements to the Visualase platform, which provides a minimally invasive surgical option for patients with focal epilepsy, brain tumors, and radiation necrosis, which impacts over 1 million people total worldwide.

Insulet (Nasdaq: PODD)+
today announced a new integration for its Omnipod 5 platform, building on its partnership with Dexcom (Nasdaq: DXCM)