CereVasc Receives Health Canada Investigational Testing Authorization for STRIDE Clinical Trial of the eShunt® System
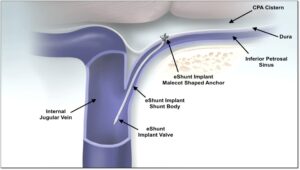
BOSTON, June 18, 2025 /PRNewswire/ — CereVasc, Inc., a clinical-stage medical device company developing novel treatments for neurological diseases, announced today that it has received Investigational Testing Authorization (ITA) from Health Canada to conduct the STRIDE trial, a clinical study evaluating CereVasc’s eShunt System as a treatment for normal pressure hydrocephalus (NPH).