SonoClear AS Receives FDA Breakthrough Designation for SonoClear® System
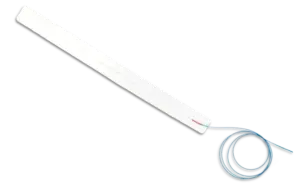
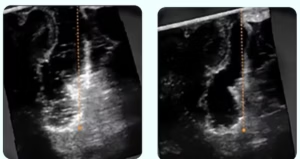
OSLO, Norway, July 1, 2025 /PRNewswire/ — SonoClear AS today announced that the Center for Devices and Radiological Health (CDRH) of the U.S. Food and Drug Administration (FDA) has designated the SonoClear® System as a Breakthrough Device for use in intracranial ultrasound procedures.