Bioengineered hydrogel mimics tumor environment to preserve live tissues for longer
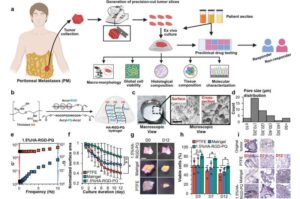
A new hydrogel-based platform to preserve live patient-derived tumor tissues in the lab could pave the way for faster, more accurate testing of cancer treatments for patients with peritoneal metastases, a hard-to-treat and often deadly form of abdominal and pelvic cancers.



