Caranx Medical surgical robot TaviPilot AI software wins FDA clearance
The FDA has granted 510(k) clearance to the TaviPilot AI software developed by Caranx Medical, according to company officials.

The FDA has granted 510(k) clearance to the TaviPilot AI software developed by Caranx Medical, according to company officials.
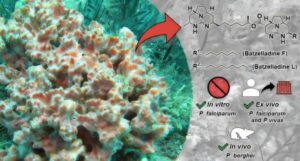
Brazilian researchers have discovered chemical compounds in marine sponges that have the potential to eliminate the malaria parasite, including strains that are resistant to conventional antimalarial drugs. The research results were published in the journal ACS Infectious Diseases.
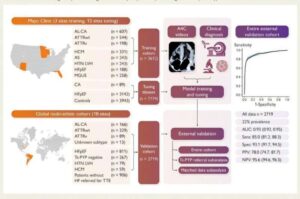
An artificial intelligence (AI) model developed by Mayo Clinic and Ultromics, Ltd., an AI echocardiography company based in Oxford, England, is highly accurate in screening for cardiac amyloidosis, a rare and progressive type of heart failure, according to a new study. The model is the first and only AI tool of its kind.
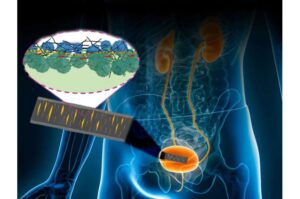
A new skin-like sensor developed by an international team led by researchers at Penn State could help doctors monitor vital signs more accurately, track healing after surgery and even help patients with bladder control issues.
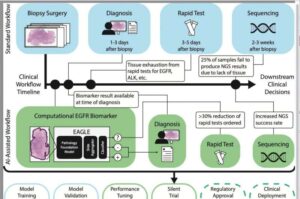
A new study by researchers at the Icahn School of Medicine at Mount Sinai, Memorial Sloan Kettering Cancer Center, and collaborators, suggests that artificial intelligence (AI) could significantly improve how doctors determine the best treatment for cancer patients—by enhancing how tumor samples are analyzed in the lab.
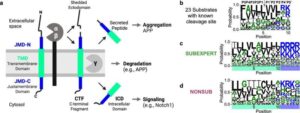
Researchers from DZNE, Ludwig-Maximilians-Universität München (LMU), and Technical University of Munich (TUM) have found that the enzyme “gamma-secretase”—implicated in Alzheimer’s disease and cancer—selects its reaction partners according to a complex scheme of molecular features.

Revolutionary Implant Integrates Sensor-Driven Technology and Cloud Connectivity to Enhance Hearing Outcomes and Patient Monitoring
Innovative Device Enhances Ease, Precision, and Accessibility for Patients Using Preservative-Free Eye Medications
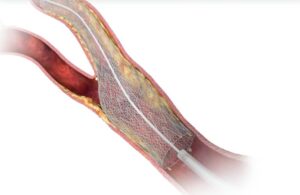
InspireMD (Nasdaq:NSPR) announced today that it launched its CGuard Prime carotid stent in the U.S. after receiving FDA premarket approval (PMA) last month.

NEUCHÂTEL, Switzerland, July 9, 2025 /PRNewswire/ — Aktiia, a pioneer in optical blood pressure monitoring, announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for over-the-counter (OTC) use of its cuffless blood pressure monitoring technology.