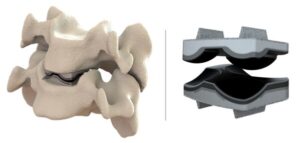
Dymicron Granted IDE Approval for Triadyme-C Artificial Disc
OREM, Utah, July 10, 2025 /PRNewswire/ — Dymicron®, a privately held medical device company pioneering advanced spinal technologies, today announced that the United States (U.S.) Food and Drug Administration (FDA) has granted Investigational Device Exemption (IDE) approval to begin a pivotal clinical study of the Triadyme®–C cervical artificial disc.
