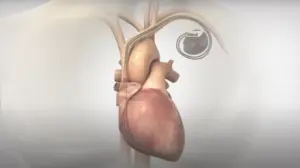
FDA approves expansion to Orchestra BioMed pacemaker trial
Orchestra BioMed (Nasdaq:OBIO) announced today that it began the rollout of a protocol update for its BACKBEAT study.

Orchestra BioMed (Nasdaq:OBIO) announced today that it began the rollout of a protocol update for its BACKBEAT study.

Nyxoah (Nasdaq:NYXH) announced today that it received FDA approval for its Genio neuromodulation device for treating sleep apnea.
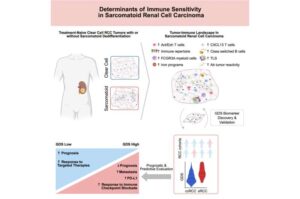
A study led by Roswell Park Comprehensive Cancer Center helps explain why a rare and hyper-aggressive subtype of kidney cancer is susceptible to immunotherapy—information that helped researchers create a first-of-its-kind tool to guide treatment decisions for advanced kidney cancers.

A mobile phone app designed to deliver suicide-specific therapy reduced suicidal behavior among high-risk psychiatric inpatients, according to a new study by scientists at Yale School of Medicine and The Ohio State University Wexner Medical Center and College of Medicine.
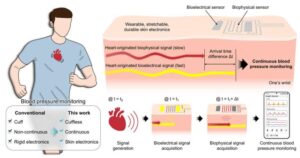
Seoul National University College of Engineering announced that a research team has developed a wearable electronic device that attaches to the skin like a bandage and enables real-time, continuous monitoring of blood pressure over extended periods.

SurgiBox, a medical technology company committed to improving access to safe, clean surgery at the point of need, announced today it has received the CE Mark under MDR for the medical devices that comprise its flagship product, the SurgiField System.

The introduction of the test is underpinned by data from the blinded, prospective EXPAND study, which began in 2023.
Under PreCheck, the FDA will communicate more frequently with pharmaceutical companies, helping them as they establish or expand manufacturing sites in the U.S.

Founded in 2009, QinFlow is a market leader in fluid thermoregulation solutions.