EPO grants patent for Autonomix Medical’s catheter technology
The patent covers a broad spectrum of applications, including arterial and renal mapping.

The patent covers a broad spectrum of applications, including arterial and renal mapping.
PERTH, Australia, Aug. 21, 2025 /PRNewswire/ — Artrya Limited (ASX: AYA) (Artrya or the Company), a medical technology company commercialising its Salix® AI-powered cloud platform, for the near real time, point of care assessment and management of coronary artery disease, is pleased to announce it has received 510(k) clearance from the U.S. Food and Drug Administration (the FDA) for Artrya’s proprietary, Salix® Coronary Plaque module.
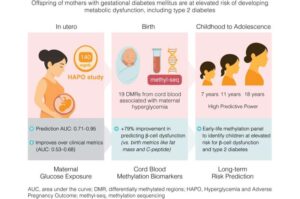
A genetic test of cord blood at birth may hold the key to predicting a child’s future risk of developing type 2 diabetes, according to exciting new research from Australia and Hong Kong.
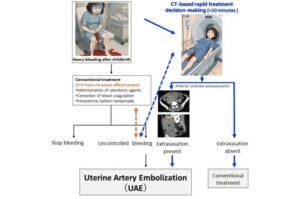
Researchers from Kumamoto University have identified a distinctive CT imaging pattern that can predict which women experiencing severe postpartum hemorrhage (PPH) are most likely to need life-saving interventions.
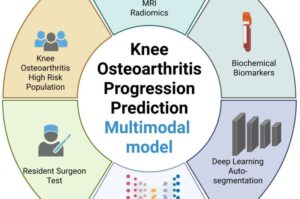
An artificial intelligence (AI)-assisted model that combines a patient’s MRI, biochemical, and clinical information shows preliminary promise in improving predictions of whether their knee osteoarthritis may soon worsen. Ting Wang of Chongqing Medical University, China, and colleagues have published this model in the journal PLOS Medicine.
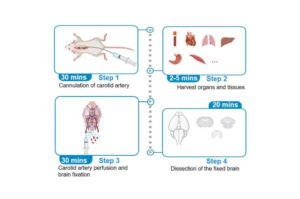
Cardiovascular researchers at UC Davis Health have developed a novel technique that allows scientists to study how the brain communicates with other organs, like the heart or gut. The new method preserves the brain tissue in animal research while simultaneously collecting living (unfixed) samples from other organs.
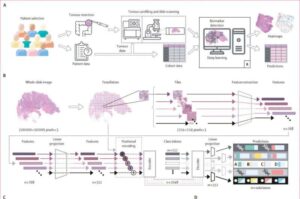
A multicenter study has analyzed nearly 2,000 digitized tissue slides from colon cancer patients across seven independent cohorts in Europe and the US. The samples included both whole-slide images of tissue samples and clinical, demographic, and lifestyle data.
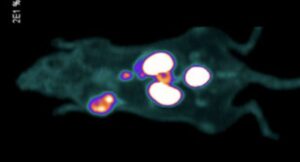
A preclinical evaluation of a new “dual-mode” tracer agent shows promise in not only helping surgeons image and plan prostate cancer procedures, but also provide them with much more consistent and targeted guidance during surgery.

Weight-loss interventions, including gastric bypass surgery and drugs that prevent dietary fat absorption, can be invasive or have negative side effects. Now, researchers have developed edible microbeads made from green tea polyphenols, vitamin E and seaweed that, when consumed, bind to fats in the gastrointestinal tract.
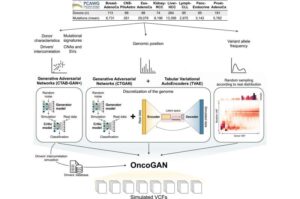
A new AI system that creates simulated cancer genomes could reshape the tools used to analyze tumors, helping bring about more accurate cancer diagnosis and ultimately more effective treatments.