Naitive receives FDA clearance for OsteoSight
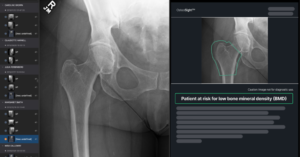
Naitive Technologies, a medical technology company developing AI-driven software to reimagine orthopedic care has announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its flagship product, OsteoSight. OsteoSight enables opportunistic assessment of bone mineral density (BMD) using standard X-rays acquired to investigate other clinical concerns, such as pain associated with arthritis or a fall.