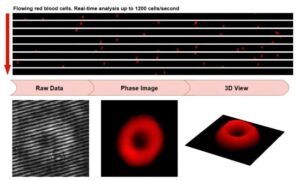
Embedded GPU platform powers real-time blood cell imaging and analysis
Blood tests are among the most common tools in medicine. Scientists are working to make blood cell imaging faster and more intuitive so that doctors can make fast and accurate diagnostic decisions.