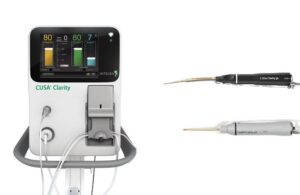
Integra wins FDA nod for ultrasonic surgical aspiratory system for cardiac surgeries
Integra LifeSciences (Nasdaq: IART)+
announced that it received FDA 510(k) clearance for its CUSA aspirator system for cardiac surgeries.

Integra LifeSciences (Nasdaq: IART)+
announced that it received FDA 510(k) clearance for its CUSA aspirator system for cardiac surgeries.

In a move for women’s health, menstrual health startup Joii is launching the world’s first AI-powered app that accurately measures period blood volume in the UK, transforming a guessing game into hard data that benefits patients and clinicians alike.

FRANKLIN LAKES, N.J., Nov. 12, 2025 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, introduces the PureWick™ Portable Collection System, a discreet, first-of-its-kind, battery-powered personal urine management device designed for wheelchair users to help improve mobility around and outside the home.

LISLE, Ill., Nov. 12, 2025 /PRNewswire/ — Hubly Surgical today announced FDA 510(k) clearance expanding Hubly Auto-Stop Drill indications to include spinal decompression procedures. To date, many thousands of neurosurgical patient lives have been saved using Hubly Auto-Stop Drills; the device’s SMART auto-stop halts rotation and prevents forward plunge at the instant of skull penetration, preventing over-drilling into patients’ brains. The new clearance expands use to laminectomy and laminotomy for spinal decompression to drill through the vertebral lamina and protect patients from damage to the spinal cord.
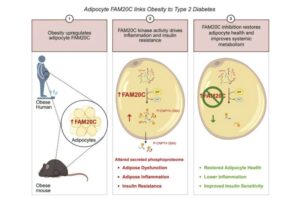
Weill Cornell Medicine investigators have identified an early step in a cellular process that leads to inflammation in fat cells and may result in type 2 diabetes in people with obesity.
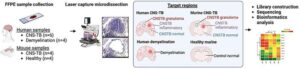
Researchers at the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine), have demonstrated that doxycycline, a commonly available and inexpensive antibiotic, can improve survival rates and neurological outcomes in central nervous system tuberculosis (CNS-TB) in a preclinical non-human study.

Researchers at Texas A&M University have uncovered a key element of joint cartilage regrowth, which brings them one step closer to regrowing human limbs.
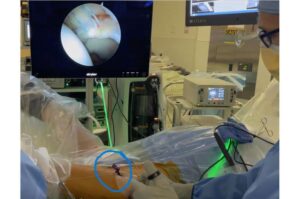
There’s a new tool in the hands of surgeons making waves in the world of hip arthroscopy.
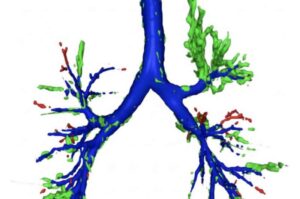
Researchers at the University of Southampton have developed an artificial intelligence (AI) tool that can spot hard-to-see objects lodged in patients’ airways better than expert radiologists.
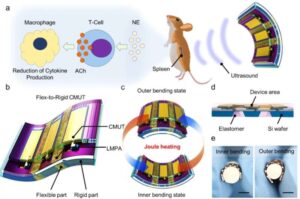
Conventional wearable ultrasound sensors have been limited by low power output and poor structural stability, making them unsuitable for high-resolution imaging or therapeutic applications.