
FDA clears Augmedics’ AR headset for use with spine surgery system
The company will introduce the headset at the North American Spine Society Annual Meeting in the US on 14 November.

The company will introduce the headset at the North American Spine Society Annual Meeting in the US on 14 November.
ViTAA’s FDA clearance on its AiORTA tool represents the first part of its planned full-suite aortic care platform.
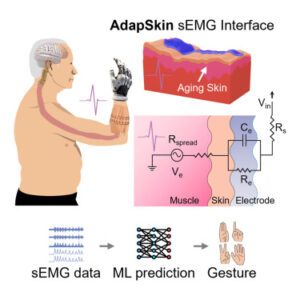
Skin-interfaced bioelectronics can help enhance health monitoring and early disease prediction. However, biomechanical and compositional differences in skin, such as dryness, wrinkling, and thinning due to the loss of collagen, can alter skin electrical impedance and conductance, compromising the functionalities of these devices.

ALTSTÄTTEN, Switzerland and BOSTON, Nov. 13, 2025 /PRNewswire/ — icotec, the pioneer of implantable devices made from BlackArmor® Engineered Carbon/PEEK, today announced that the U.S. Food and Drug Administration (FDA) has cleared the CMORE® CT System for use in the cervicothoracic spine.
NIRIT, Israel, Nov. 13, 2025 /PRNewswire/ — Smart Sound Ltd., a cochlear implant innovator, announces publication of a U.S. Patent for a “Hidden Cochlear Implant System with an In-Canal Wireless Transmission,” a novel cochlear implant design fundamentally change the industry by providing a fully discreet, magnet-free and improving users quality of life.

BREA, Calif., Nov. 13, 2025 /PRNewswire/ — Rapid Nexus Nanotech Wound Solutions, Inc., a California-based med-tech company focused on advanced wound care, today announced it has received FDA 510(k) clearance for its Hemastyl gel device—marking a historic milestone as the first company to directly target the underlying reason chronic wounds fail to heal.

In every functional MRI scan, after the whir and pounding begins, there is a brief 10 to 20 seconds of stabilization as the machine’s magnetic field settles into place. For decades, scientists have treated this period as dead time, discarding the data or “dummy scans.”
There are more candidates on the waitlist for a liver transplant than there are available organs, yet about half the time a match is found with a donor who dies after cardiac arrest following the removal of life support, the transplant must be canceled.
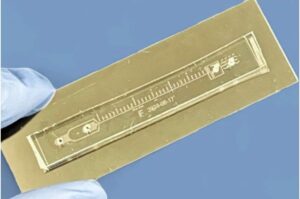
Pinned between the stomach and spine, the pancreas supervises both digestion and blood sugar in the body. It’s also the site of an aggressive cancer called pancreatic ductal adenocarcinoma, or PDAC.