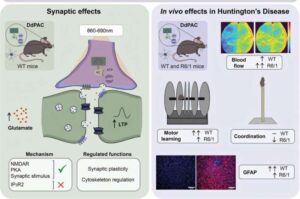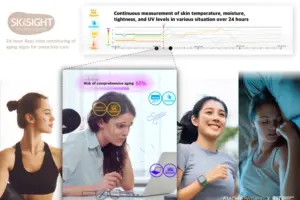
SkinSight™ AI by ModuleMD: Smarter Skin Analysis, Faster Clinical Decisions
GRAND BLANC, Mich., Dec. 10, 2025 /PRNewswire/ — ModuleMD, ranked as one of America’s fastest-growing companies on the Inc. 5000 and top-rated EHR vendor on G2, announces SkinSight™ AI, a solution that brings automation, accuracy, and consistency to one of the most manual and variable workflows in allergy care: skin test measurement and documentation.