
MannKind earns FDA nod for on-body infusor, new autoinjector patents
MannKind (Nasdaq:MNKD) announced today that the FDA approved its Furoscix (furosemide) on-body infusor for pediatric patients.

MannKind (Nasdaq:MNKD) announced today that the FDA approved its Furoscix (furosemide) on-body infusor for pediatric patients.

A new method could enable users to design portable medical devices, like a splint, that can be rapidly converted from flat panels to a 3D object without any tools.

Edwards Lifesciences (NYSE: EW)+
has won FDA approval for its Sapien M3 mitral valve replacement system for treating mitral regurgitation (MR).

Shape Memory Medical’s IMPEDE embolisation plug range has received CE mark as a Class III device under the European Union’s Medical Device Regulation (EU MDR).

A multicenter study led by UC Davis Health has tested a new treatment designed to improve care for people with a rare liver disease called primary sclerosing cholangitis. Researchers learned that an anti-inflammatory and anti-fibrotic monoclonal antibody known as nebokitug was safe and showed potential efficacy in patients with PSC.
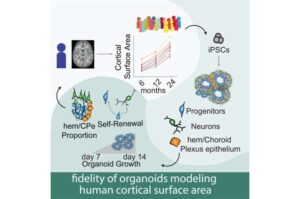
A new study in the lab of Jason Stein, Ph.D., modeled brain development in a dish to identify cells and genes that influence infant brain growth, a trait associated with autism.