BrainSpace wins FDA clearance for automated brain fluid management device
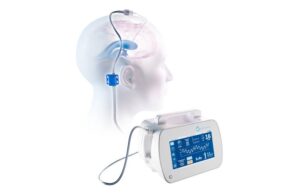
BrainSpace announced that the FDA granted 510(k) clearance for its Intellidrop automated brain fluid management system.

BrainSpace announced that the FDA granted 510(k) clearance for its Intellidrop automated brain fluid management system.

Ceribell (Nasdaq:CBLL) announced today that the FDA granted breakthrough device designation for its large vessel occlusion (LVO) stroke detection monitor.
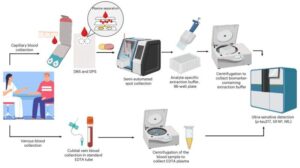
A new international study has demonstrated that Alzheimer’s disease biomarkers can be accurately detected using simple finger-prick blood samples that can be collected at home and mailed to laboratories without refrigeration or prior processing.
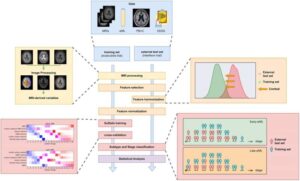
Artificial intelligence (AI), using a simple blood test combined with standard brain images has, for the first time, been able to identify two biologically distinct types of multiple sclerosis (MS).

Researchers at the Halle branch of the Fraunhofer Institute for Cell Therapy and Immunology IZI have identified a substance that selectively blocks harmful pathogens such as Porphyromonas gingivalis without affecting other bacteria.
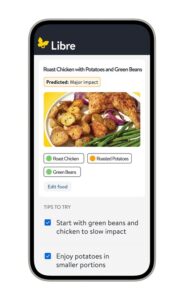
ABBOTT PARK, Ill., Jan. 5, 2026 /PRNewswire/ — Abbott (NYSE: ABT), a leading healthcare company, today unveiled Libre Assist,1 a groundbreaking feature within the Libre app5 designed to help the millions of people living with diabetes in the U.S. better understand how the foods they eat affect their glucose levels. 1,2 Unlike traditional food logging apps that only give feedback after a meal is logged, Libre Assist1 helps people make informed mealtime decisions before they eat. Abbott is launching the new technology during CES 2026 in Las Vegas.