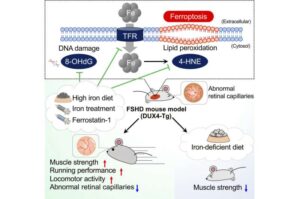
Iron supplements restore muscle strength in mouse model of muscular dystrophy
Researchers at Kumamoto University have demonstrated that iron supplementation can significantly alleviate muscle pathology and functional decline in a mouse model of facioscapulohumeral muscular dystrophy (FSHD), a rare genetic muscle disease for which no effective treatment currently exists.