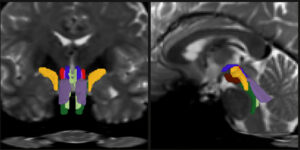
AI algorithm enables tracking of vital white matter pathways
Opening a new window on the brainstem, a new tool reliably and finely resolves distinct nerve bundles in live diffusion MRI scans, revealing signs of injury or disease.

Opening a new window on the brainstem, a new tool reliably and finely resolves distinct nerve bundles in live diffusion MRI scans, revealing signs of injury or disease.
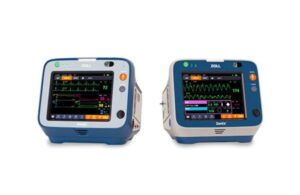
Zoll announced today that it received CE mark approval under the European Medical Device Regulation (MDR) for its Zenix monitor/defibrillator.

PLANO, Texas, Feb. 10, 2026 /PRNewswire/ — Vesalio, a global leader in vascular intervention, today announced CE Mark certification and the European commercial launch of two new neurovascular devices: NeVa™ VS, for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage (aSAH), and the NeVa™ 3.0 mm Thrombectomy System for stroke. In addition, the Company received an additional U.S. Food and Drug Administration (FDA) 510(k) clearance expanding the indications of its neurovascular and peripheral aspiration catheters to include distal access.

Researchers at the University of Arkansas have developed an artificial-intelligence diagnostic tool to detect masked hypertension.
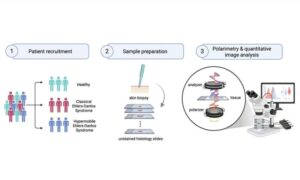
In a recent study by researchers in Toronto (Canada), scientists tested an optical method called Mueller matrix polarimetry to address a diagnostic gap.

Engineers at Stanford University have developed a high-efficiency, battery/solar-operated, autonomous microscope with integrated artificial intelligence that automatically diagnoses malaria in blood smears

Research on their new robotic crash cart (RCC)—a robotic version of the mobile drawer unit that holds supplies and equipment needed for a range of medical procedures.
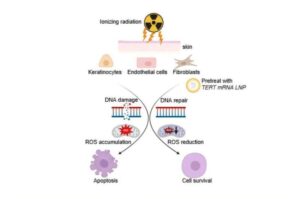
Researchers at Houston Methodist Research Institute have now discovered a promising new approach that can protect patients from radiation-induced skin damage during cancer treatment.
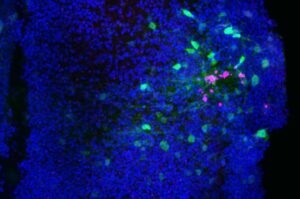
In the future, more targeted strategies that dampen damaging, chronic oxidative stress while preserving—or even harnessing—these short-lived pro-healing signals could open new avenues to promote brain repair.
Artificial intelligence could help doctors detect serious heart valve disease years earlier, potentially saving thousands of lives, a new study suggests.