
Encora wins FDA clearance for wearable device for essential tremor
Encora Therapeutics announced today that it received FDA 510(k) clearance for its Encora X1 device.

Encora Therapeutics announced today that it received FDA 510(k) clearance for its Encora X1 device.

Novocure (Nasdaq:NVCR) announced that the FDA granted approval for its Optune Pax treatment for advanced pancreatic cancer.

BRECKSVILLE, Ohio, Feb. 12, 2026 /PRNewswire/ — Applied Medical Technology, Inc. (AMT) today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Explant Express®, a breast implant removal device designed to support efficient, controlled explantation of ruptured silicone breast implants.

A multidisciplinary study led by the Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) and published in Circulation Research provides a new perspective on why this arrhythmia can persist long-term, highlighting the key role of non-contractile cardiac cells.
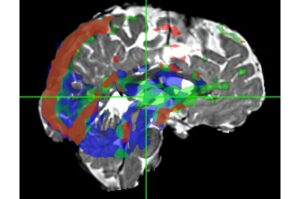
A new MRI technique called Velocity Spectrum Imaging can map fluid movement in the human brain within a 3D pixel.
With a new MRI technique that shows both heart tissue and blood flow simultaneously, physicians can see where heart defects occur and precisely plan to repair them, according to new research.
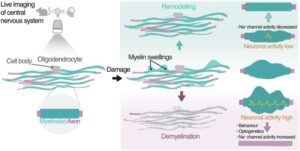
An international research team has gained new insights into the dynamics of myelin swellings in the brain.
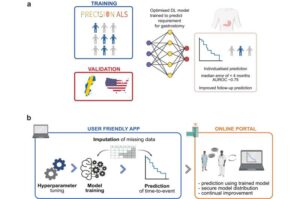
A new AI tool that accurately predicts the need for a feeding tube could transform patient care and improve quality of life for people living with Motor Neuron Disease (MND).
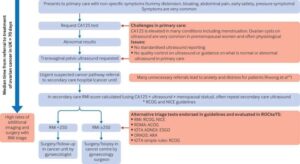
The IOTA ADNEX ultrasound tests picked up 9 out of 10 women with cancer.

For the first time ever, NTNU researchers have identified new characteristics of aggressive prostate cancer.