
Philips introduces flexible pediatric MRI coil
Philips (NYSE: PHG)+ announced that it launched the InkSpace Imaging Snuggle pediatric body array coil for its 3.0T MRI systems.

Philips (NYSE: PHG)+ announced that it launched the InkSpace Imaging Snuggle pediatric body array coil for its 3.0T MRI systems.

Winning approval to target the 15,000 U.S. patients with locally advanced pancreatic cancer is the first step in a broader expansion in the tumor type.

HERAKLION, Greece, Feb. 13, 2026 /PRNewswire/ — EYE PCR announces that its fixOflex endocapsular device has received CE Mark certification under the European Union Medical Device Regulation (EU MDR 2017/745), enabling commercialization in Europe and other markets that recognize the CE Mark. This regulatory milestone validates the device’s safety and efficacy, positioning EYE PCR for controlled market introduction.
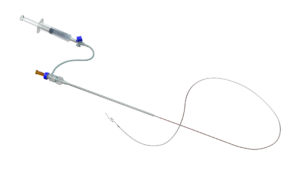
GALWAY, Ireland, Feb. 13, 2026 /PRNewswire/ — InVera Medical, an Irish medical technology company, has received European regulatory approval for a new minimally invasive device designed to help physicians deliver treatment more effectively to diseased leg veins, including varicose veins.
TAICHUNG, Feb. 13, 2026 /PRNewswire/ — Cancer has long ranked among the leading causes of death in Taiwan, and solid tumors account for more than 90% of cancer cases worldwide—making them the most challenging frontier for CAR-T cell therapy. China Medical University Hospital (CMUH) announced today that, in collaboration with Ever Supreme Bio Technology, it has successfully developed the world’s first EXO 001 targeted exosome platform, a breakthrough technology that enables direct in vivo programming of T cells to generate multi-target nanobody-based CAR-T cells.
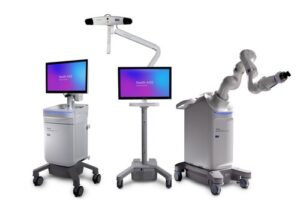
GALWAY, Ireland, Feb. 13, 2026 /PRNewswire/ — Medtronic (NYSE: MDT), a global leader in healthcare technology, today announced U.S. Food and Drug Administration (FDA) clearance of the Stealth AXiS™ surgical system, a next-generation platform that brings planning, navigation, and robotics together into a single, intelligent system for spine surgery.

New techniques to detect Y chromosome genes show frequent loss of the Y in tissues of older men.
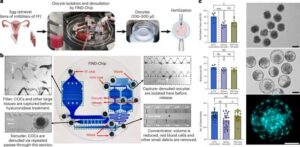
Scientists have now developed a way to find missing eggs that could bring new hope to couples seeking fertility treatment.